
Digestive efficiency is one of those reptile misconceptions that makes the rounds every now and again. It’s not as pervasive as the “lack of aerobic capacity” or “inability to maintain body temperature” arguments, but there is still a general view in many scientific circles (*cough* paleontology *cough*) that reptiles are less efficient at digesting food than similar sized mammals and birds. Much of this boils down to the old endothermocentric fallacy that the high costs associated with obligate endothermy should somehow translate to greater benefits everywhere else (Greenberg 1980).
Well that, and the fact that reptile chewing is very different from mammalian chewing.
Oral processing: To chew or not to chew
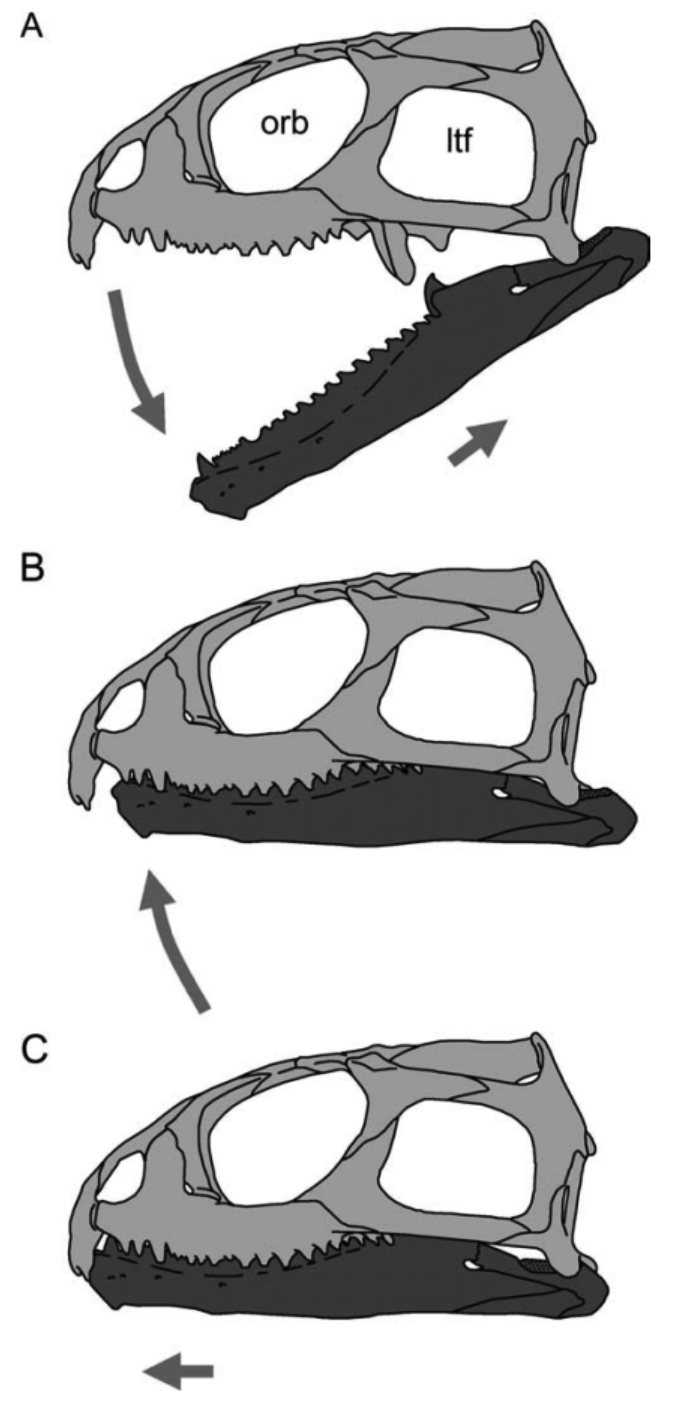
This is a big sticking point in mammalian studies. For decades, mammals were considered the only animals that chewed. It was viewed as a synapomorphy of Mammalia, as they went from the “simple” oral processing of reptiles, to the complicated mastication observed in extant mammals (e.g., Mills 1972).
This view started to change a bit in the late 1960’s with the discovery of dinosaur species that housed extensive tooth batteries (ceratopsians and ornithopods), indicative of some appreciable level of oral processing (e.g., Ostrom 1961, Bakker 1986). Much more recently, it has come to light that many other extant reptiles also chew, such as Uromastyx, Sphenodon, and all tortoises (Throckmorton 1980, Gorniak et al. 1982, Gerhlach 1999, Schwenk et al. 2000, Reilly et al. 2001, Jones et al. 2012). Perhaps most shocking of all is evidence that at least once species of snake also chews (Kojima et al. 2020).
All of these reptiles (save maybe the snake), and probably all of the dinosaurs listed earlier evolved a method of chewing that involves shoving their mandibles forward relative to their cranium at the end of each bite. This proal motion creates a shearing force between the upper and lower teeth that helps chop food into smaller pieces (this is often referred to as propalinal in the literature, but see Nabavizadeh’s take on this terminology here). As with mammals, all of these reptiles swap food to different sides of the jaw when chewing, allowing for more force to be applied to the ingested food.
This swapping of jaw sides can best be seen in caiman lizards (Dracaena guianensis) which regularly feed on hard-shelled prey such as snails, and will spend a large amount of time just cracking that shell open to eat the gooey innards.
Discoveries such as this eventually led to the walking back of “chewing is just for mammals” and now things like chewing and oral processing are considered widespread among animals. Now, mammalogists argue for maintaining “mastication” as a mammal-only trait (Ross et al. 2007), which…I don’t really support, but I can at least understand the reasoning.
The long and short of it is that many reptiles chew. However, the extent of their oral processing is still pretty limited compared to the extensive chewing (mastication) seen in mammals (save for D. guianensis. That little dude is doing some work).
This difference in degree of chewing leads to differences in the size of the ingested material. Food particles from mammalian chewers are much smaller than similar-sized food particles from a reptilian chewer. These smaller particles have increased surface areas for digestive enzymes to work on them, allowing for a faster breakdown of that food. Thus, mammals should be better a digesting than a similar-sized reptile.
Carnivores need not apply
Everything I have mentioned here does carry an important caveat. All of these discussions on digestive efficiency mostly revolve around herbivorous animals. Carnivores are considered efficient digesters regardless of the species. Animal parts are a more ready-made state for breaking down nutrients than plants are (thanks to the cellulose-based cell walls that need breaking through). So, an insectivore and a meat eater should have approximately the same degree of digestive efficiency. The largest distinction here will be in how much of the animal gets ingested. For instance, Komodo dragons and leopards will digest the same amount of ingested meat at approximately the same efficiency, but the dragons will eat more of the prey item than the leopard, so they will ultimately utilize more of the carcass (Auffenberg 1981, Chakrabarti et al. 2016).
Herbivory is really what drives extensive and complicated chewing. Plant material is difficult to access and low in macronutrients. Herbivores need to either be selective in what they eat, supplement with occasional animal (often insect) bits, or eat a tonne of low-quality plant material and digest as much of it as possible.
Mechanical vs. microbial digestion
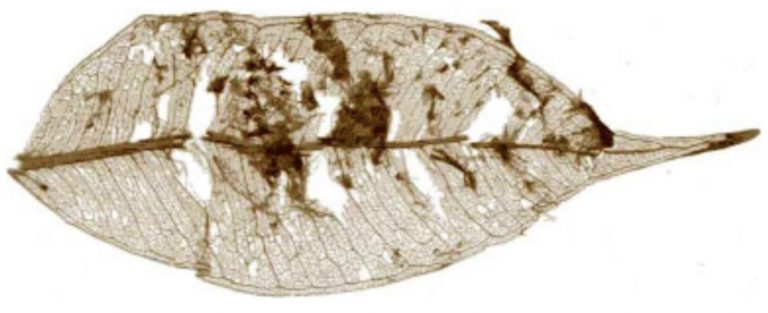
As I laid out earlier, mammals chew. Herbivorous mammals chew a whole lot. Their extensive oral processing breaks down plant material into smaller pieces that allow digestive enzymes to infiltrate more of the plant and do their work extracting nutrients from the food. In contrast, herbivorous reptiles only chew their food a little bit before swallowing. The initial oral processing does still break the plant food into slightly smaller pieces, but most of the tooth damage only creates holes and tears to allow enzymes to enter more of the plant. So, larger food items ultimately wind up going through the digestive system. However, by the end of their trip through the GI tract, ingested particle size is far reduced from when they initially entered the body. This is thanks to retention in the low PH stomach, and long transit times through the intestines, with increased retention in the often well-developed hindgut.
As it turns out, both of these methods work equally well (more on that below). The microbial digestion of reptiles takes longer, since the food items are larger, but the end results are still the same. The final, excreted waste may look more familiar, but the level of digestion in that waste turns out to be about the same (Fritz et al. 2010).
What the new study shows
This latest study is a review of previous work on reptilian digestion:
Wehrle, B.A., German, D.P. 2023. Reptilian Digestive Efficiency: Past, Present, and Future. Comp Biochem Physiol A. 277:111369.
Wehrle and German reviewed 93 studies on reptile digestion across all major branches of extant Reptilia except for Sphenodontia. Apparently, there have yet to be any digestion studies done on tuataras. Along with surveying what species were studied, the authors looked at how these studies were performed and what they measured. The most important measurement for digestion studies are:
- Apparent Digestive Efficiency / Coefficient (ADE / ADC)
- Food measured before it has been eaten and after (i.e., poop) with the acknowledgement that some extra material aside from undigested food (e.g., intestinal lining) will also be in that poop or in those urates.
- Apparent Metabolizable Energy Coefficient (MEC)
- The percentage of ingested food energy that does not leave the body as a waste product (feces and urates). This is also known as assimilation efficiency.
Of the two, MEC is a more accurate measure of available energy obtained from a food item. As expected, this also makes it harder to measure as you effectively need to burn a sample of the food and the waste material to see what the caloric differences are. In contrast, ADE is just a weight measurement (often dry mass), which can be done quickly and easily. Thankfully, both work fine if the goal is to just see how much food gets assimilated into the body, and thus how efficient the digestion of that material is.
Wehrle and German’s review provides a great summary of our current understanding of reptile digestion. Below are some of the key takeaways that I was able to glean from it.
Lizards are overstudied

At least, compared to all other reptile groups. Wehrle and German’s paper is only twelve pages long (not counting the reference section), yet the lizard section covers slightly more than half of those twelve pages. The authors discuss digestibility of animal material (insects), plant material, commercial diets, mixed diets, differences in digestibility between obligate insectivores, omnivores, and obligate herbivores. There are studies looking at difference in digestive efficiency with age, evolutionary split (some taxa are young enough that they can be compared to their ancestral populations), and of course, temperature. Lizards are the poster children for reptile digestion studies. Most of what we know about reptile digestive potential comes from these studies, and the limitations of these studies have coloured our perceptions of reptilian digestion overall (more on that below).
We can thank croc farms for crocodylian digestion studies
Okay, so this isn’t all that surprising. As soon as crocodylians were shown to be commercially viable it made sense that there would be a financial incentive for understanding how to best feed them so they can reach large (harvestable) sizes as fast as possible. Crocodylians—across the board—show high digestive efficiencies of any meat fed to them. Their powerful gastric juices make quick work of most material passing through their gastrointestinal tracts.

Two of the more interesting takeaways from crocodylian dietary studies are that they:
- Are very susceptible to high-fat diets. While all macronutrients are absorbed at high rates, crocodylian physiology really likes to grab fats and store them away. This is what initially led to alligator obesity rates at alligator farms.
- Crocodylians readily eat plant material. While this has been known to happen in passing, many previous observations were chalked up to incidental ingestion. It wasn’t until Staton et al. (1990) that it became clear that many crocodylians will happily eat plant food. They don’t ingest it often enough to be considered omnivores, but they do ingest it more often than one would expect for a hypercarnivore. Hileveski et al. 2022 provides a thorough review of our current knowledge of this behaviour.
Stressed animals have poorer digestion than relaxed animals
Yeah, this one seems like a given, right? Despite this, Wehrle and German discovered that several digestion studies relied on force-feeding food to the animals. Force feeding usually meant prying open the mouths of the animals and stuffing a syringe full of broken-up food bits into the esophagus.
Why did so many studies do this?
The likely reasoning behind it has to do with the typical behaviour of most reptiles (nay, most animals) when stressed. They just shut down their appetites and reallocate energy away from the digestive system until the situation feels more comfortable. While this behaviour is pretty common for most animals, the low resting metabolic rates of reptiles and their tendency to select for lower than optimal temperatures during these stressful times enhances the duration of their fast. If the animals are herbivores, then the delayed energy acquisition from the breakdown of their last meal prior to the stressful situation will further enhance this length of time. An ugly truth behind most reptile “minimum food requirement” observations is that they usually came from animals that were held in stressful, captive situations that allowed the animals to starve to death. This is how we know that large crocodylians can last over a year without eating. Just because they can do it, doesn’t mean they normally do.
Stressed animals take time to adjust to their new surroundings. Unfortunately for researchers, that acclimatization time may exceed the available length of the study / funding window. This time crunch has apparently led several researchers to resort to force-feeding of their animals, which just adds to animal stress and makes a compromised digestive system work even worse. In their literature survey, Wehrle and German found over 1/4th of the studies they reviewed relied on force-feeding their animals. Results of many force-feeding trials showed noticeably lower digestive efficiencies that were likely due to stress mixed with the higher frequency of meals.
Digestive efficiency is independent of temperature
I’m adding in a caveat here. This finding was true to an extent. A reptile that is well below its preferred temperature range may completely shut down its GI tract, which could result in that food just sitting there and rotting in place. More than likely the animal will just expelled this food from its body (i.e., vomit) which avoids that problem, but also means that digestive efficiency is close to nil since nothing is really “on” at this point. Similarly, reptiles that are well-above their preferred body temperature are likely to suffer from catastrophic physiological problems that make digestion the least of their worries.
Aside from these dangerous extremes, almost all of the studies that Wehrle and German looked at showed that temperature had negligible to no effect on digestive efficiency. A python digesting a rabbit at 32°C will do so just as well as a python digesting that rabbit at 17°C. The only thing that changes is the transit time through the gut. Warmer animals push food through their guts faster. This independence of digestive efficiency is fascinating as it implies that the animals must be adjusting their enzymatic activity to account for the changing rate of transit of the food. There is some self-balancing that takes place here as enzymatic activity rises with temperature, so digestive efficiency will naturally match food passage rate without the aid of a conformation change (i.e, an allosteric effect), though such a match will not be exactly 1:1. A similar association should occur at lower temperatures too, as enzymes become less active and transit time through the gut increases. It’s the exceptions to this apparent rule that suggest that there is more going on under the hood than simple thermodynamics. Some species, such as Trachylepis margaritifera show faster gut transit at lower temperatures while retaining the same digestive efficiency (Miller et al. 2014). This suggests that there are at least some reptile species that do adjust enzyme affinity to offset reduced reaction rates, though this hypothesis has yet to be tested.

There are a lot of confounding variables that make comparisons difficult
This was the largest take-home message of the paper. Wehrle and German did their best to do an apples-to-apples comparison of the data, but more often then not they found that an accurate comparison was not possible as the required information was just not reported / measured.
Most reported data was “flat” percentages, which are easy to understand and can be readily compared across taxa. Unfortunately, their simplicity is also their biggest problem. As Wehrle and German mention in the paper, energy intake is a shared variable of the numerator and denominator in these digestive efficiency equations. This means that the errors on both sides of these fractions will be the same, which can lead to spurious correlations (Raubenheimer 1995) that can artificially prop up the efficiency of the digestion equations or find a statistically relevant correlation that is not actually there.
Beaupre and Dunham (1995) re-analyzed their earlier published ADE and MEC data for Sceloporous merriami (Beaupre et al., 1993) and identified that using energy consumption as a covariate instead of a ratio denominator decreased MEC by as much as 12 percentage points (71.6% in ratio-only analysis to 59.5% using ANCOVA). This substantial decrease in MEC revealed that a 10-day energy budget using the ratio-based estimation would overestimate the energy available to the lizard by >20%!
This suggests that current estimates of animal digestive efficiency may be overestimating just how well well these animals are able to extract energy from their food. On the bright side, this is a global problem. So, if we have been overestimating reptile digestive efficiency, we’ve been doing the same about mammals, birds, fish, insects, etc. These errors won’t all trend the same way, but they will all be there. Unfortunately, this does make cross-taxa comparisons more difficult to do.
Final take home
Wehrle and German have provided a much needed review of the current state of reptile digestibility studies.
Their data solidifies what has been mentioned in multiple studies (but for some reason easily missed), reptiles are just as good as mammals and birds when it comes to digestion of food. At least from a higher order taxon point of view. In reality, digestive efficiency studies are going to vary by individual species, with some performing better than others. It’s only with these more global perspectives that the individual variation flattens out to a more consistent signal. In this case, that signal is parity with every other taxonomic class.
Discovering that so many reptile digestion studies required force feeding really makes me skeptical of future studies that come out comparing digestive efficiency. In a similar vein, discovering the inherent flaws of all digestion studies means that we need to be more careful about cross-species comparisons. Especially when that cross-species spans orders and classes of animals.
All in all this is a good paper to have handy for any future biologist or paleontologist that is looking to understand energetic differences between different animal groups.
~Jura
References
Auffenberg, W. 1981. The Behavioral Ecology of the Komodo Monitor, Florida University press.
Bakker, R. 1986. The Dinosaur Heresies: New Theories Unlocking the Mystery of the Dinosaurs and their Extinction. William Morrow. New York.
Beaupre, S.J., Dunham, A.E. 1995. A comparison of ratio-based and covariance analyses of a nutritional data set. Funct Ecol 9:876–880.
Beaupre, S.J., Dunham, A.E., Overall, K.L. 1993. Metabolism of a desert lizard: the effects of mass, sex, population of origin, temperature, time of day, and feeding on oxygen consumption of Sceloporus merriami. Physiol Zool 66:128–147.
Chakrabarti, S., Jhala, Y.V., Dutta, S., Qureshi, Q., Riaz F. Kadivar, R.F., Vishwadipsinh J. Rana, V.J. 2016. Adding constraints to predation through allometric relation of scats to consumption. J. Animal. Ecol. 85(3): 660-670.
Fritz, J., Hummel, J., Kienzle, E., Streich, W.J., Clauss, M. 2010. To chew or not to chew: Fecal particle size in herbivorous reptiles and mammals. J. Exp. Zool. 313A:579–586.
Gerlach, J. 1999. Feeding behavior and the saddleback shell of Dipsochelys arnoldi. Chelonian Cons. Biol. 3(3):496–500.
Gorniak, G.C., Rosenberg, H.I., Gans, C. 1982. Mastication in the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): Structure and activity of the motor system. J. Morph. 171:321–353.
Greenberg, N. 1980. “Physiological and Behavioral Thermoregulation in Living Reptiles” in: A Cold Look at the Warm-Blooded Dinosaurs (R.D.K. Thomas and E.C. Olson Eds.), pp. 141-166, AAAS, Washington, DC.
Hileveski, S., Cordero, T., Siroski, P., 2022. Do crocodilians eat plant material? A review of plant nutrients consumed by captive crocodilians. S Am J Herpetol 24: 19–25.
Jones, M.E., O’Higgins, P., Fagan, M., Evans, S.E., Curtis, N. 2012. Shearing mechanics and the influence of a flexible symphysis during oral food processing in Sphenodon (Lepidosauria: Rhynchocephalia). Anat. Rec. 295:1075–1091.
Kojima, Y., Fukuyama, I., Kurita, T., Mohamad, Hossman, M.Y.B., Nishikawa, K. 2020. Mandibular sawing in a snail-eating snake. Sci. Rep. 10:12670.
Miller, A.K., Erasmus, B.F.N., Alexander, G.J. 2014. Digestive efficiencies are independent of gut passage times in rainbow skinks (Trachylepis margaritifer). Comp Biochem Physiol A 175:110–114.
Mills, J.R.E. 1972. Evolution of Mastication. Proc R Soc Med. Vol 65:392-396.
Ostrom, J.H. 1961. Cranial Morphology of the Hadrosaurian Dinosaurs of North America. Bull AMNH. Vol. 122(2):33–186.
Reilly, S.M, McBrayer, L.D, White, T.D. 2001. Prey processing in amniotes: Biomechanical and behavioral patterns of food reduction. Comp. Biochem. Physiol. A. 128:397–415.
Ross, C.F., Eckhardt, A., Herrel, A., Hylander, W.L., Metzger, K.A., Schaerlaeken, V., Washington, R.L., Williams, S.H. 2007. Modulation of intra-oral processing in mammals and lepidosaurs. Int. Comp. Biol. 47(1):118-136.
Schwenk, K. 2000. “Feeding in lepidosaurs” in: Feeding: Form, Function, and Evolution in Tetrapod Vertebrates. ed: Schwenk, K. pp:175–291. San Diego, CA. Academic Press.
Staton, M.A., Edwards, H.M., Brisbin, L., Joanen, T., Mcnease, L., 1990. Protein and energy relationships in the diet of the American Alligator (Alligator mississippiensis).J Nutr 120: 775–785.
Throckmorton, G.S. 1980. The chewing cycle in the herbivorous lizard Uromastix aegyptius (Agamidae). Arch. Oral. Biol. 25:225–233.

I would love to soon see you talk about the evolution of scales and filaments in diapsids (especially in dinosaurs) and dispel some (WAY to prominent) myths like “scales makes dinosaurs too monstrous” (they don’t) or “filaments ancestorial to all ornithodirans (I’m less sure about how to approach this one because I have believed this SINCE I was about 8-9 years of age but I am able to change my worldview on the evolution of filaments in this group and filaments evolving multiple times would actually be really cool and really does not make a dent in the bird-dinosaur connection). Also could the scales on dinosaurs interact with water droplets like some lizards do or no? Just curious.